1. Boryl reagents for organic synthesis, small molecule activaction and catalysis.
We are interested in the design and synthesis of organoboron compounds, which have potential applications in organic synthesis, small molecule activaction and catalysis.
4) Diborencine Macrocycle
Encapsulation of a single electron within the internal cavity of a host system poses significant challenges, as the electron tends to delocalize over the surface. In this study, we successfully trap a single electron through a B–B one-electron σ-bond in a diborencine macrocycle (compound 4). The structure of this one-electron σ-bond has been characterized using X-ray single-crystal analysis and EPR studies, indicating that this bond exhibits considerable s-character, with the boron atoms adopting sp3 hybridization, as supported by DFT computations. Additionally, compound 4 demonstrates rich reactivity: it can facilitate O2 cleavage, generating a B–O–B cyclic product; its reaction with PhSSPh produces a B–S–B linked ring-expansion product, while reactions with PhSeSePh and quinone yield ring-contraction products. The resulting products have been fully characterized through X-ray single-crystal analysis, NMR, and HRMS spectroscopy. Finally, DFT computational studies have been performed to elucidate the reaction mechanism of this one-electron B–B bond.
Y. Wu, Y. Pan, J. Yao, G. Xu, K. Lau, and Z. Lu. Encapsulation of an Electron in a Diborencine Macrocycle: Synthesis, Structure, and Reactivity. J. Am. Chem. Soc., 2025, DOI: 10.1021/jacs.5c12718.
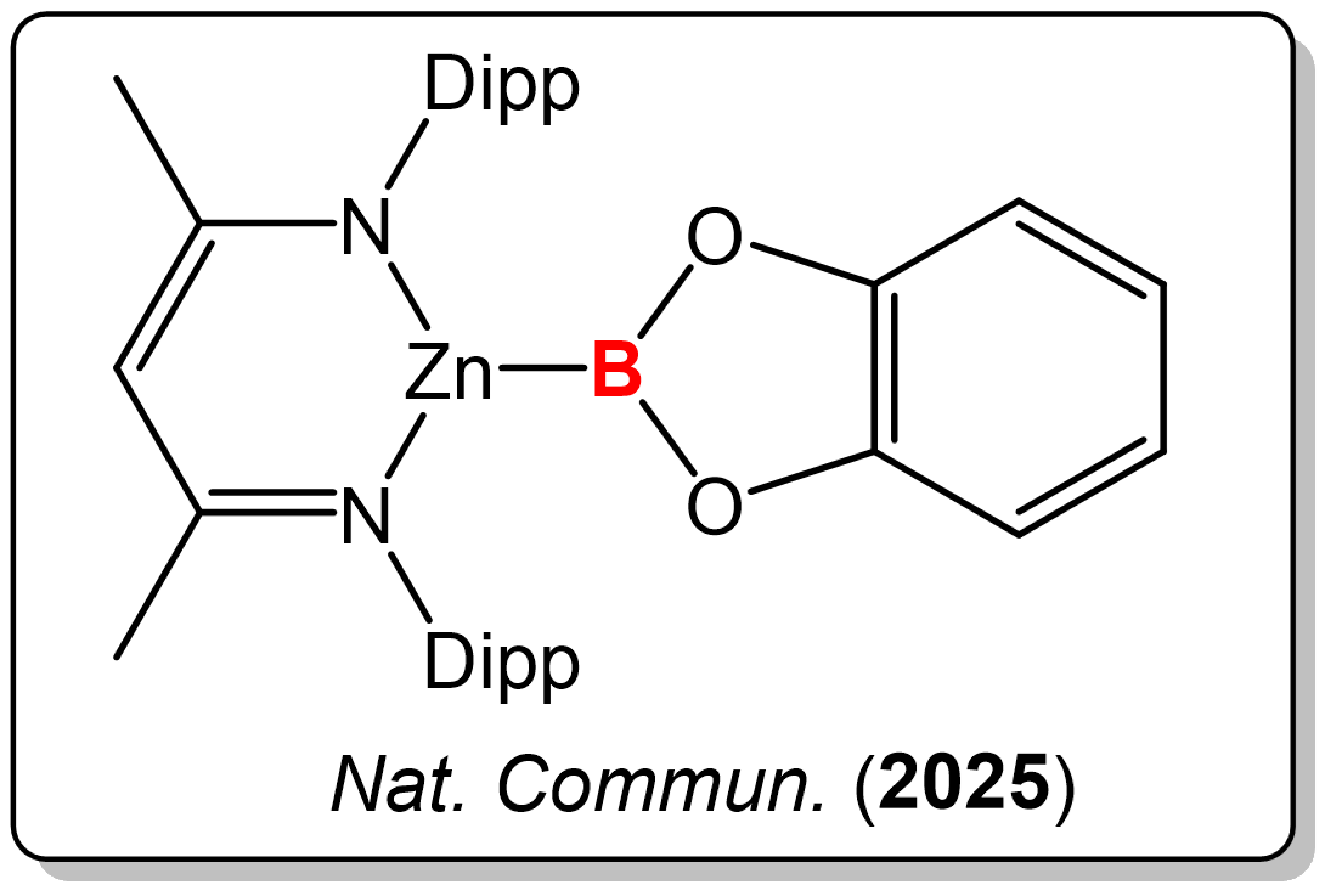
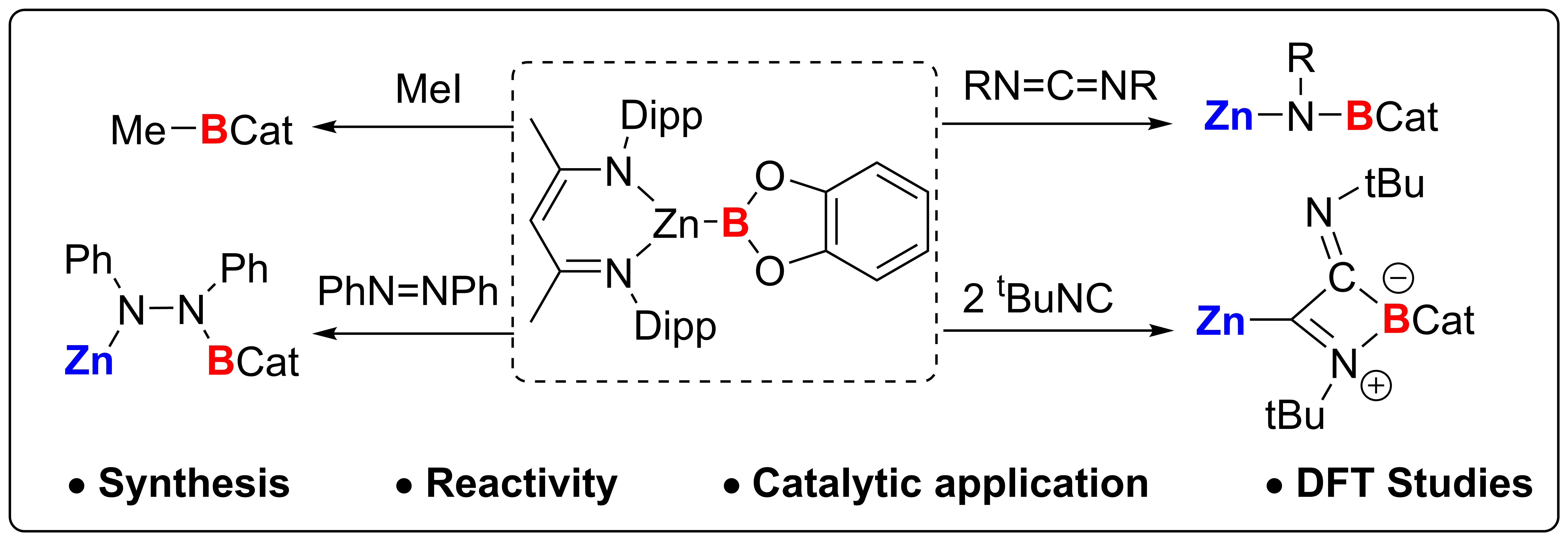
3) Zinc-Boryl Species Reactivity
Borylation chemistry plays a crucial role in the development of new synthetic methodologies. However, the reactivity of zinc-boryl species has not been fully explored, particularly in relation to diverse reaction pathways. Here we show that a zinc-boryl species is successfully synthesized from bis(catecholato)diboron, exhibiting amphiphilic reactivity. This compound acts as a nucleophilic boron anion with methyl iodide and as an electrophile with N,N'-dicyclohexylcarbodiimide, facilitating zinc-boron bond dissociation and generating zinc-carbon and zinc-nitrogen bonds while cleaving carbon-nitrogen double bonds. The enhanced reactivity is likely due to the stronger covalency of the zinc-boron bond. Additionally, the zinc-boryl compound promotes the catalytic diborylation of azobenzene, underscoring its versatility as a reactive intermediate. Density functional theory studies illuminate the electronic structure and reactivity of the zinc-boron bond, providing insights into potential applications in synthetic chemistry.
G. Xu, H.T. Chan, S. Li, T.Y. Wong, L. Zhang, Q. Zhang, Z. Lin and Z. Lu. A zinc boryl compound unlocks diverse reactivity pathways beyond nucleophilic borylation. Nat. Commun., 2025, 16, 6349.
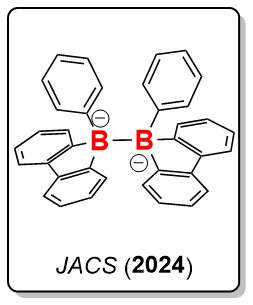
2) Boryl Radicals through B–B Bond Cleavage
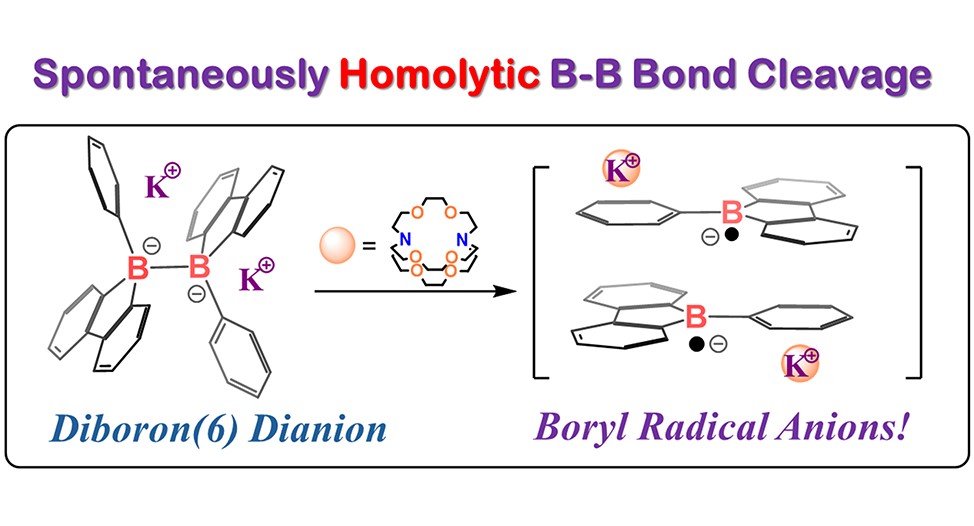
Our study unveils a novel approach to accessing boryl radicals through the spontaneous homolytic cleavage of B–B bonds. We synthesized a hexaaryl-substituted diboron(6) dianion, 1, via the reductive B–B coupling of 9-borafluorene. Intriguingly, compound 1 exhibits the ability to undergo homolytic B–B bond cleavage, leading to the formation of boryl radical anions, as confirmed by EPR studies, in the presence of the 2.2.2-cryptand at room temperature. Moreover, it directly reacts with diphenylacetylene, producing an unprecedented 1,6-diborylated allene species, where the phenyl ring is dearomatized. Density functional theory computational studies suggest that homolytic B–B bond cleavage is favored in the reaction path, and the formation of the boryl radical anion is crucial for dearomatization. Additionally, it achieves the dearomative diborylation of anthracene and the activation of elemental sulfur/selenium under mild conditions. The borylation products have been successfully characterized by NMR spectra, HRMS, and X-ray single-crystal diffraction.
S. Li, F. Shiri, G. Xu, K.S.M. Yiu, H. K. Lee, T. H. Ng, Z. Lin and Z. Lu. Reactivity of A hexaaryldiboron(6) Dianion as Boryl Radical Anions. J. Am. Chem. Soc., 2024, 146, 17348-17354.
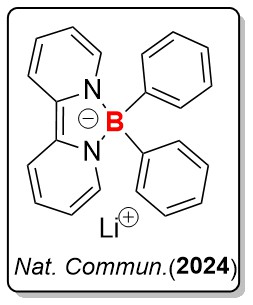
1) Redox-Active of Borate Anions
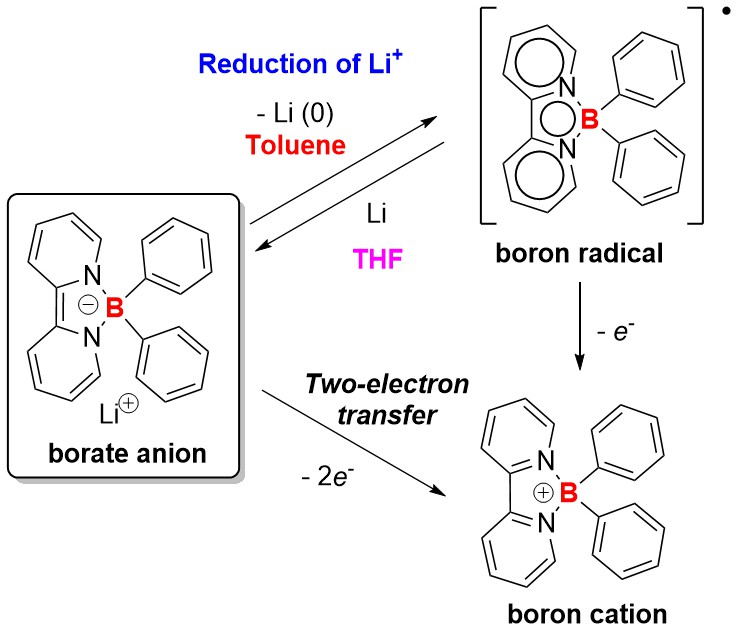
Group 1 elements exhibit the lowest electronegativity values in the Periodic Table. The chemical reduction of Group 1 metal cations M+ to M(0) is extremely challenging. Common tetraaryl borates demonstrate limited redox properties and are prone to decomposition upon oxidation. In this study, by employing simple yet versatile bipyridines as ligands, we synthesized a series of redox-active borate anions characterized by NMR and X-ray single-crystal diffraction. Notably, the borate anion can realize the reduction of Li+, generating elemental lithium metal and boron radical, thereby demonstrating its potent reducing ability. Furthermore, it can serve as a powerful two-electron-reducing reagent and be readily applied in various reductive homo-coupling reactions and Birch reduction of acridine. Additionally, this borate anion demonstrates its catalytic ability in the selective two-electron reduction of CO2 into CO.
H. Li, J. Yao, G. Xu, K.S.M. Yiu, C. Siu, Z. Wang, Y. Peng, Y. Xie, Y. Wang, Z. Lu. Reduction of Li+ within A Borate Anion. Nat. Commun., 2024, 15, 2590. (Highlighted in Communications Chemistry.)